- Overview
- Facility
- Process Development
- Manufacturing
- ADC Platform
- Analytical Service
Overview
LOTTE BIOLOGICS operates a cGMP ADC manufacturing facility in the U.S., offering comprehensive one-stop ADC services.
Our Syracuse Bio Campus is equipped for both clinical and commercial ADC production, leveraging over 18 years of expertise in antibody
manufacturing to deliver optimized ADC solutions tailored to the diverse needs of global clients.
ADC (Antibody Drug Conjugate)
An ADC (Antibody-Drug Conjugate) is a next-generation anticancer therapy that combines an antibody with a chemically synthesized drug to eliminate cancer cells.
Why LOTTE BIOLOGICS
LOTTE BIOLOGICS is pioneering new possibilities in targeted therapeutics through ADC technology while also providing comprehensive ADC CDMO services.

- 01
Establishing a state-of-the-art ADC facility
at the Syracuse Bio Campus, one of the
premier ADC sites in North America.
(2025) - 02
Equipped with production facilities
ranging from 100L to 1,000L.
(single-use reactors) - 03
Expertise in handling
high-potency APIs - 04
Target Occupational Exposure Limit (OEL): 10ng/m³
(Minimizing hazardous substance exposure
and enhancing safety)
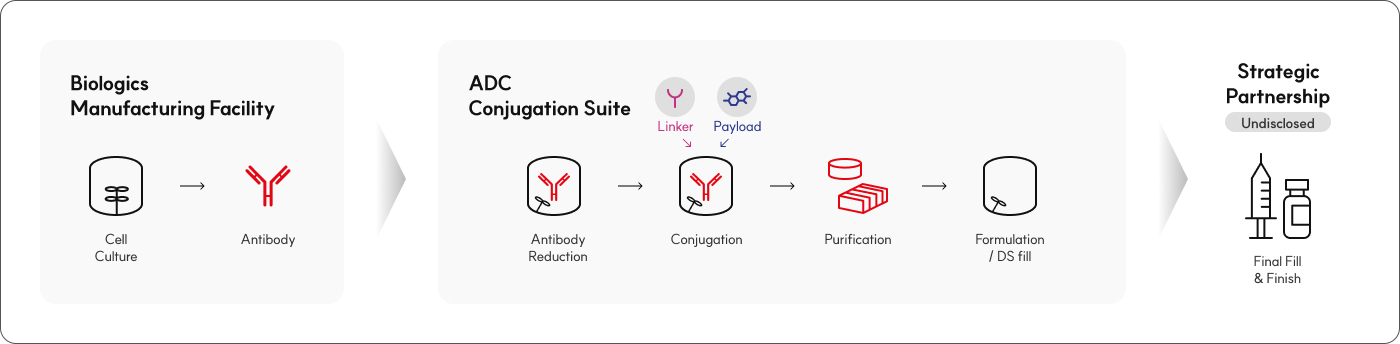
One-stop ADC CDMO service
LOTTE BIOLOGICS provides one-stop ADC services at its Syracuse Bio Campus in the U.S., covering everything from cell line development and
antibody production to conjugation.
Available Services

Bioconjugation Development
- Process development
- Analytical method development
- ADC platform toolbox (SoluFlex Link™)
Bioconjugation Manufacturing
- non-GMP/cGMP ADC DS Manufacturing
Analysis
- QC
- Characterization
ADC Platform
LOTTE BIOLOGICS is strengthening its competitiveness in the ADC field and proactively responding to market demands through strategic partnerships
with leading ADC technology companies.

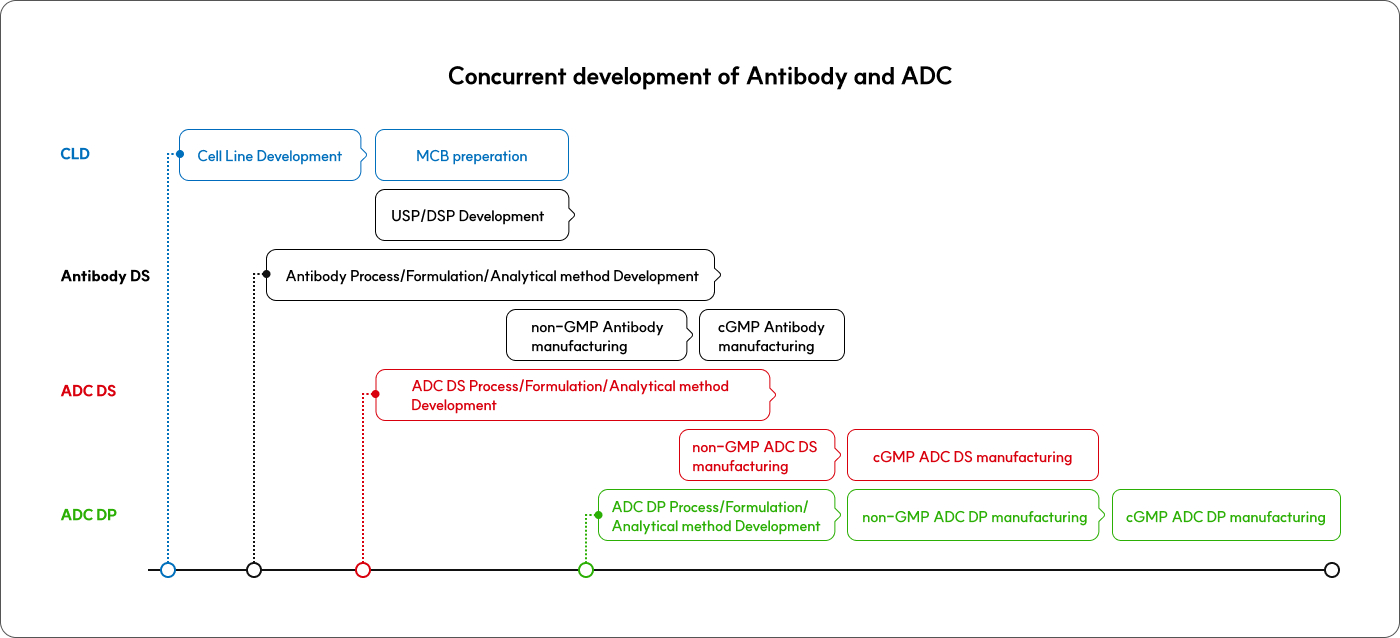
Concurrent development of Antibody and ADC
By seamlessly coordinating simultaneous development from cell line development to ADC DP (through partnerships),
we enhance development speed and efficiency.
Available Modalities *Including but not limited to the examples below:
- ADC/BsADC
- Antibody-Oligonucleotide Conjugate
- Antibody-Chelator Conjugate
- Degrader Antibody Conjugate
- Immuno-Stimulant Antibody Conjugate
- Antibody-Peptide Conjugate
- PEGylation-Based Conjugate
- Antibody-Glycan Conjugate
- Antibody-Fluorophore Conjugate
Strategic Partnerships
LOTTE BIOLOGICS is collaborating with leading companies possessing advanced ADC technology to secure competitiveness in the ADC sector and proactively respond to market demands.


